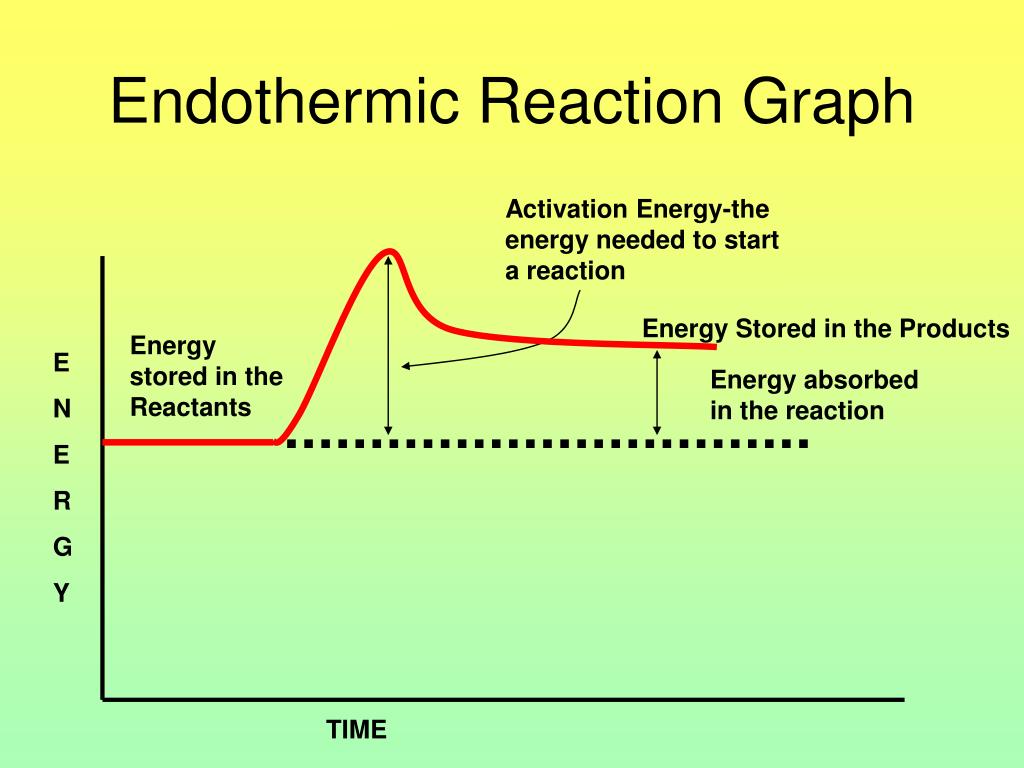
Endothermic Reactions Are Defined By A . In the course of an. The heat is absorbed from the surroundings allowing the reactants to. Define endothermic and exothermic reactions. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. Determine if a chemical process is exothermic or endothermic. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Use or interpret energy diagrams. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat.
from www.slideserve.com
In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. Determine if a chemical process is exothermic or endothermic. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. In the course of an. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. Use or interpret energy diagrams. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Define endothermic and exothermic reactions. The heat is absorbed from the surroundings allowing the reactants to.
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint
Endothermic Reactions Are Defined By A Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Use or interpret energy diagrams. In the course of an. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. Define endothermic and exothermic reactions. Determine if a chemical process is exothermic or endothermic. The heat is absorbed from the surroundings allowing the reactants to. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat.
From mmerevise.co.uk
Endothermic and Exothermic Reactions Revision MME Endothermic Reactions Are Defined By A A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Define endothermic and exothermic reactions. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings. Endothermic Reactions Are Defined By A.
From techiescientist.com
Is Condensation Endothermic or Exothermic? Techiescientist Endothermic Reactions Are Defined By A Define endothermic and exothermic reactions. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. Use or interpret energy diagrams. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. In the course of an. The heat is absorbed from the surroundings allowing the. Endothermic Reactions Are Defined By A.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reactions Are Defined By A Determine if a chemical process is exothermic or endothermic. The heat is absorbed from the surroundings allowing the reactants to. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. In the. Endothermic Reactions Are Defined By A.
From ar.inspiredpencil.com
Endothermic Reaction Equation Endothermic Reactions Are Defined By A An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. In the course of an. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. The heat is absorbed from the surroundings allowing the reactants to. Define. Endothermic Reactions Are Defined By A.
From www.fity.club
Comparing Endothermic And Exothermic Potential Energy Endothermic Reactions Are Defined By A Define endothermic and exothermic reactions. The heat is absorbed from the surroundings allowing the reactants to. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. In endothermic and exothermic. Endothermic Reactions Are Defined By A.
From www.slideserve.com
PPT Endothermic Vs. Exothermic Reaction Graphs PowerPoint Endothermic Reactions Are Defined By A Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. Use or interpret energy diagrams. Define endothermic and exothermic reactions. Determine if a chemical process is exothermic. Endothermic Reactions Are Defined By A.
From gy.kimiq.com
In An Exothermic Reaction Energy Is Transferred From Energy Etfs Endothermic Reactions Are Defined By A The heat is absorbed from the surroundings allowing the reactants to. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Determine if a chemical process is. Endothermic Reactions Are Defined By A.
From www.diffzy.com
Exothermic vs. Endothermic Reaction What's the Difference (With Table) Endothermic Reactions Are Defined By A The heat is absorbed from the surroundings allowing the reactants to. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. Use or interpret energy diagrams. In endothermic and exothermic reactions, energy can be. Endothermic Reactions Are Defined By A.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reactions Are Defined By A An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. In the course of an. The heat is absorbed from the surroundings allowing the reactants to. Define endothermic and exothermic reactions. Determine if a chemical process is exothermic or endothermic. Use or interpret energy diagrams.. Endothermic Reactions Are Defined By A.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reactions Are Defined By A Use or interpret energy diagrams. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. The heat is absorbed from the surroundings allowing the reactants to. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Determine. Endothermic Reactions Are Defined By A.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Reactions Are Defined By A Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer. Endothermic Reactions Are Defined By A.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Reactions Are Defined By A A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Use or interpret energy diagrams. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a. Endothermic Reactions Are Defined By A.
From www.aiophotoz.com
Endothermic Reaction Diagram Labeled Diagram Media Images and Photos Endothermic Reactions Are Defined By A Use or interpret energy diagrams. In the course of an. In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to. Endothermic Reactions Are Defined By A.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Endothermic Reactions Are Defined By A Define endothermic and exothermic reactions. The heat is absorbed from the surroundings allowing the reactants to. In the course of an. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. A chemical reaction or physical change is endothermic if heat is absorbed by the. Endothermic Reactions Are Defined By A.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Reactions Are Defined By A Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. Determine if a chemical process is exothermic or endothermic. Define endothermic and exothermic. Endothermic Reactions Are Defined By A.
From www.memory.com
Question Endothermic reaction Memory Endothermic Reactions Are Defined By A A chemical reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. A chemical reaction is said to be endothermic when it absorbs energy, mostly heat. Endothermic reactions are chemical reactions in. Endothermic Reactions Are Defined By A.
From study.com
Endothermic Reaction Definition & Example Video & Lesson Transcript Endothermic Reactions Are Defined By A In endothermic and exothermic reactions, energy can be thought of as either a reactant of the reaction or a product. Use or interpret energy diagrams. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. In the course of an. The heat is absorbed from the surroundings allowing. Endothermic Reactions Are Defined By A.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Reactions Are Defined By A An endothermic reaction is a chemical change in which the system absorbs thermal energy from its surroundings resulting in the overall increase in its total. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their surroundings. Define endothermic and exothermic reactions. The heat is absorbed from the surroundings allowing. Endothermic Reactions Are Defined By A.